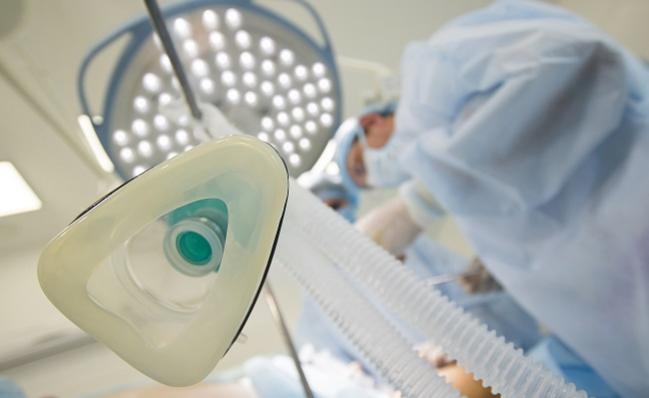
Thanks to donors to LHSF's COVID-19 Response Fund, along with grants through the provincial government and the Canadian Institute for Health Research (CIHR), a new trial based out of Lawson Health Research Institute is hoping to address a potential global shortage of IV sedatives used prior to ventilating patients.
“While IV sedatives are currently used, there is concern about global drug shortages, particularly if there is a second wave of COVID-19 in the fall,” explains Dr. Marat Slessarev, Scientist at Lawson and Critical Care Physician at London Health Sciences Centre (LHSC).
.jpg)
This study will determine whether inhaled sedatives can replace those delivered intravenously in patients with COVID-19 requiring ventilation. Inhaled sedatives are widely available due to their use in operating rooms, yet they are not routinely used for patients requiring ventilation. Their potential use for COVID-19 patients presents a rapidly scalable solution that could immediately ease pressures on IV sedative stocks and enable safe ventilation.
Inhaled sedatives can also put less strain on the body, leading to better patient outcomes. Previous research into the use of inhaled sedatives for patients requiring ventilation showed reduced lung inflammation, improved oxygen levels, shortened duration of ventilation and ICU stays, and signs of improved survival and recovery.
Almost all patients who survive their ICU stay are left with gaps in their comprehension and understanding (cognition). While some patients recoup full cognition, many patients, especially marginalized populations without social supports, will suffer irreversible impairment. Given that cognitive function is one of the best predictors of life quality, work success, social enjoyment, happiness, and life-expectancy, it is important to identify which sedation strategy will generate better cognitive outcomes.
Led by LHSC and Sunnybrook Health Sciences Centre, the study will recruit approximately 800 patients from across Canada and the U.S. Each patient will be randomized to receive either IV sedatives or inhaled sedatives. Clinicians will compare survival, length of ventilation and cognitive outcomes between the two groups. If the results favour inhaled sedatives, this will transform the standard of care for ventilated patients and save health care systems around the world valuable resources and money that can be put towards other urgent needs (each individual IV sedation kit costs $30-35 compared to $2-5 for inhaled sedatives).
“This is the largest trial of its kind. If inhaled sedatives can shorten the length of ventilation or improve survival in patients with severe respiratory failure, this could cause a paradigm shift in the way we sedate patients in ICUs around the world,” notes Dr. Slessarev.
This critical project was awarded a first-place ranking by CIHR against 438 national proposals, and was bolstered significantly by generous donations.
“We are very grateful for the kind support of our local donors, who have provided funds for the purchase of critical equipment for our ICUs to enable this trial. With their gift, LHSC is in a great position to lead this important and innovative trial.”
To give to the project or learn more, contact douglas.macrae@lhsc.on.ca or donate today to the COVID-19 Response Fund.
OTHER DONOR-FUNDED COVID-19 RESEARCH
First study to identify potential therapeutic targets for COVID-19
A non-invasive mask for ventilation
A global trial to fast-track treatment
A human protein with great treatment potential
